Starting September 6, 2024, a trial scheme has been implemented, where submission of documents in English for new drug approval applications is accepted by PMDA (related article). Here, we would like to clarify once again what a “new drug” is under the Pharmaceuticals and Medical Device Act.
Definition of “New Drug”
The PMDA website describes the definition of new drug as follows (Japanese only):
“New drugs” are drugs that are obviously different from already approved drugs in terms of active ingredients, amount, usage, dosage, efficacy, effects, etc., as stipulated in Article 14-4 of the Pharmaceuticals and Medical Devices Act, and mainly include drugs containing new active ingredients, new medical combination drugs, drugs with new administration routes, drugs with new efficacy, drugs with new dosage forms, and drugs with new dosages.
As the PMDA explains, a drug that is “obviously different” from an already approved drug falls under the category of a “new drug.”
It’s easy to imagine that drugs with different active ingredients are called “new drugs.”
Meanwhile, a drug can also be called a “new drug” if, for example, a new indication or new dosage or administration is approved under the Pharmaceuticals and Medical Device Act.
Legally, “new drugs” are defined in Article 14-4, Paragraph 1, Item 1 of the Pharmaceuticals and Medical Devices Act as follows:
pharmaceuticals instructed by the Minister of Health, Labour and Welfare upon approval as those that have active components, quantities, directions, dosage, efficacy and effects, etc. which are obviously different from those of pharmaceuticals which have already been approved pursuant to the provisions of Article 14 or Article 19-2 (hereinafter referred to as “new pharmaceuticals”)
Article 14-4 of the Pharmaceuticals and Medical Devices Act is a provision regarding the setting of a re-examination period. A drug for which a new re-examination period is set (by the instruction of the Minister of Health, Labour and Welfare) can also be considered as a “new drug.”
Standard premarket review process for “new drug” in Japan
This information is posted on the PMDA website (here).
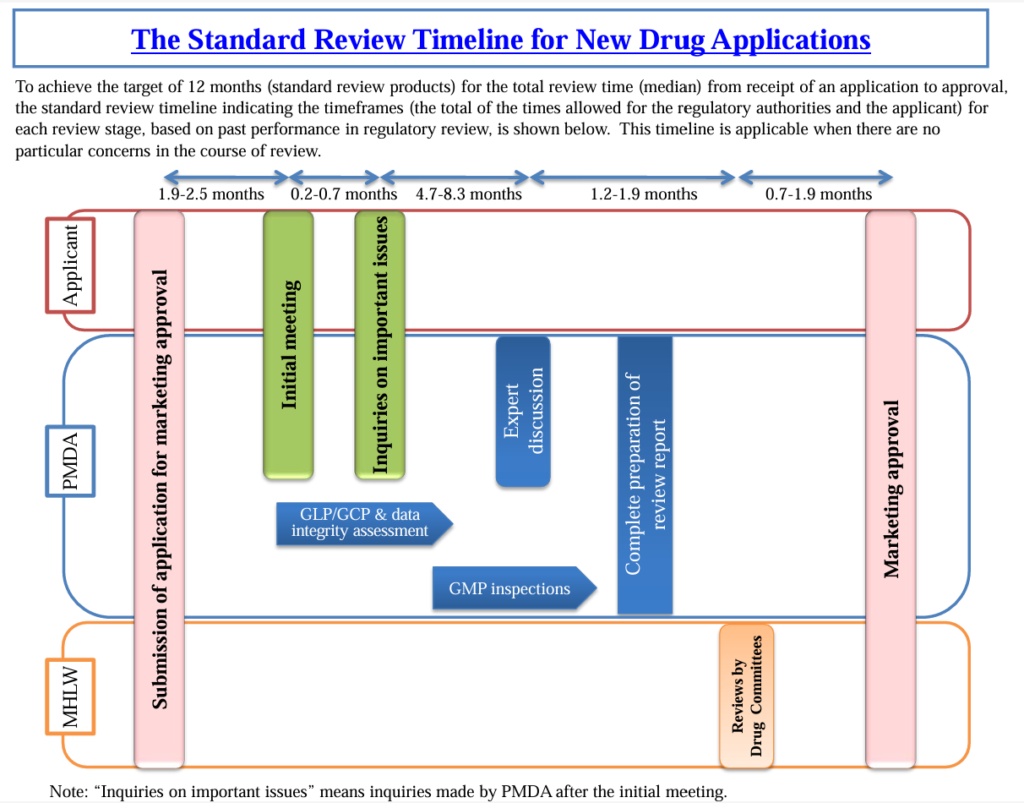
The above is for new drugs undergoing standard pre-market review. For a new drug with standard pre-market review, its target total review period (PMDA’s review period + period required by its applicant to respond to inquiries, etc.) is 12 months.
In case of new drugs undergoing priority pre-market review, its target total review period is 9 months. New drugs designated as Orphan Disease Drugs are eligible for priority pre-market review.
In both cases of standard and priority review, the initial meeting with the PMDA is held, followed by the inquiries on important issues approximately two months after the approval application. In some cases, the initial meeting is not held, where the inquiries on important issues are provided a little earlier.
In addition, the period from Drug Committee to approval is essentially the same for standard and priority reviews. Actions after Drug Committee are uniformly handled by the MHLW, not the PMDA.
Compared to new drugs with priority review, those with standard review have more time between the issuance of inquiries on important issues and Expert Discussion. The target is to complete the GCP/GLP & Data Integrity Assessment (inspection) before Expert Discussion and the GMP inspection before Review Report is completed or Drug Committee is held. Therefore, these inspections also have a little more time for new drugs with standard review.
For each new drug, the expected timeline of each event during pre-market review (i.e. initial meeting, inquiries on important issues, Expert Discussion, Drug Committee, inspections) is communicated by the PMDA through a “pre-consultation meeting for pre-market review timeline” (free of charge) held before the approval application. (the notice regarding pre-consultation meeting for pre-market review timeline is here only in Japanese) The pre-consultation meeting for pre-market review timeline is expected to be held 3 to 1 month before the approval application (according to PMDA’s guidelines for pre-consultation meeting for pre-market review timeline of new drug: only in Japanese).
It is noted that the documents required for approval applications are shown in the notice “Regarding Drug Approval Applications” (dated November 21, 2014, Director-General of the Pharmaceuticals and Medical Devices Bureau; only in Japanese). Here too, the specification for “new drug” is described.
Related Link
- Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (Pharmaceuticals and Medical Devices Act): https://www.japaneselawtranslation.go.jp/ja/laws/view/3213


コメント