An administrative notice entitled “Submitting documents to be attached in the application for approval of new ethical drugs” was issued on September 6, 2024, indicating that it is possible to submit documents in English for new drug approval applications in Japan. (Effective immediately)
This is a simple administrative notice, a short one-page notice, but as it is the first step in a major change, I would like to summarize the information.
Background of the issuance of the administrative notice
The administrative notice states that this measure is “to make it easier for foreign business entities to submit a new drug approval application in Japan in order to eliminate drug lag and loss.”
The PMDA response to consultations and pre-market reviews in English has been also mentioned in the interim report by Council of the Concept for Early Prevalence of the Novel Drugs to Patients by Improving Drug Discovery Capabilities (Summary, Full Report). In addition, the roadmap to take measures mentioned in the interim report clearly states that a trial scheme to accept English documents by PMDA will begin in FY2025, as follows:
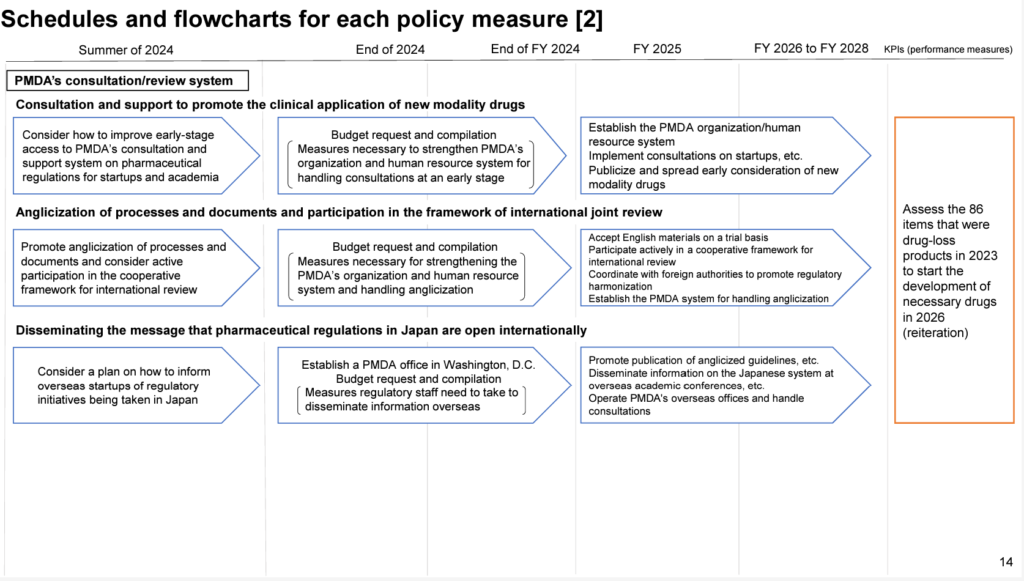
Based on this assumption, the MHLW’s budget request for FY2025 also includes a corresponding measure. This is the “Early Pharmaceutical Consultation and Support Project to Strengthen Drug Discovery Capabilities,” which provides PMDA consultations in English for seeds certified by the Japanese Government. Translation costs have been included in the budget. (Click here for a summary of information on the FY2025 budget request by the MHLW, though only Japanese.)
Based on this assumption, the MHLW’s budget request for FY2025 also includes a corresponding measure. This is the “Early Pharmaceutical Consultation and Support Project to Strengthen Drug Discovery Capabilities,” which provides PMDA consultations in English for seeds certified by the Japanese Government. Translation costs have been included in the budget. (Click here for a summary of information on the FY2025 budget request by the MHLW, though only Japanese.)
Meanwhile, “Global Health Vision of Ministry of Health, Labour and Welfare of Japan” issued on August 26, 2024 by the MHLW also includes a separate statement that “With future expansion of the ecosystem in mind, we will move forward with accepting the entire application dossier in English for the market approval of ethical drugs in Japan.”
Given these circumstances, it is surmised that further acceleration to accept documents in English were discussed (top-down) in the process of creating the Global Health Vision.
Conditions for submitting documents in English
The following conditions apply to the submission of documents in English based on this administrative notice, which is on a trial basis.
- It must be an application for approval of a “new drug.”
- “At the time of application”, all contents of the CTD, including the approval application form and package insert, can be written in English.
- It must be an application for approval by a “foreign company without an office/branch in Japan.”
The first point, “new drug,” does not mean that all drugs ( or other products such as medical devices and ATMPs) are covered. The PMDA website describes the definition of new drug as follows:
“New drugs” are drugs that are obviously different from already approved drugs in terms of active ingredients, amount, usage, dosage, efficacy, effects, etc., as stipulated in Article 14-4 of the Pharmaceuticals and Medical Devices Act, and mainly include drugs containing new active ingredients, new medical combination drugs, drugs with new administration routes, drugs with new efficacy, drugs with new dosage forms, and drugs with new dosages.
The second point can be read as meaning that all documents may be written in English at the time of application. In other words, the possibility that Japanese documents may be required by a certain point (by the time of approval) cannot be ruled out.
The third point is a condition that is easy to understand. However, the notice also specifies that, if you plan to submit documents in English, you must consult with the PMDA Review Management Department in advance. I think that one challenge will be whether the PMDA Review Management Department can communicate appropriately and in a timely manner with foreign companies that meet this condition.
Others
First, regarding the second point above, the indication that documents in English might possible only at the time of new drug approval application comes from Article 283 below in Regulation for Enforcement of the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices:
(Indication in Japanese) Article 283: Any written application, notification, report, or other documents submitted to the Minister of Health, Labour and Welfare, the Director of the Regional Bureau of Health and Welfare, a prefectural governor, the mayor of a city with established health centers or the head of a special ward in the case where the location is in a city or a special ward with a public health center must be written in Japanese; provided, however, that this does not apply to documents where some special occasion prevents indication in Japanese and a translation is attached.
The provision of the Regulation requires that at least approval applications be written in Japanese (or translated). Since the contents of the Regulation cannot be changed by administrative notices, this article continues to apply.
In addition, although the CTD contains information on the draft package insert of a drug. When the drug is finally distributed in Japan, the package insert must be written in Japanese (according to Article 218 of the Regulation).
Therefore, the wording in the administrative notice is the result of an attempt to proceed with the Japanese Government commitment as quickly as possible while complying with these provisions of the Regulation.
Next, it is stated that the trial scheme will be continued “for the time being,” and that the possibility of expanding it will be considered after that. It is not at all clear from this administrative notice how long this “for the time being” is expected to last.
On the other hand, if MHLW and PMDA are serious about expanding the acceptance of documents in English, it is possible that the provisions of the Regulation mentioned above will be addressed. In that sense, considering that discussions are currently underway to revise the Pharmaceutical and Medical Device Act, and that a bill to revise the Pharmaceutical and Medical Device Act is expected to be submitted this fall or next year (2025), such timings may make it clear whether they are really expanding this scheme by revising these provisions or not.


コメント