The timing of drug approval is an important factor that affects the outlook for development schedules (application timing, approval timing, drug price listing timing) and the preparations for drug launch.
On April 24, 2024, the Director of the Pharmaceutical Evaluation Division issued a notice entitled “Regarding the Approval Timing of New Drugs,” showing the policy regarding the timing of new drug approvals in Japan. This article outlines the contents of the notice and explains the specific expected schedule. (the Notice in Japanese here)
In addition, following the issuance of this notice, at the 589th General Assembly of the Central Social Insurance Medical Council (Chuikyo) on May 15, 2024 (materials in Japanese), it was explained that the drug price listing period will change from four times a year to seven times a year from 2025. (Related article in Japanese only)
The operation based on this notice is to be applied to new drugs that are discussed/reported at the Drug Committees in January 2025 or later.
Please also refer to here for the definition of “New Drug”.
Content of the Notice
The notice clearly says that new drug approval is to be made within approximately three weeks after the relevant Drug Committee, although there is room for specific adjustments product by product.
However, for new drugs that are discussed/reported at the Drug Committees held between October and December of the year prior to the revision of medical treatment fees (drug price revision as well), the approvals for these drugs are to be made within approximately three weeks of the last Drug Committee during that period. That is, new drugs discussed in Drug Committees in October, November and December are to be approved in December at all once.
In addition, the notice indicates that the timing of approval of new drugs that are discussed/reported at the Drug Committees in fiscal year 2026 (from April 1, 2026) may be reconsidered.
Estimated schedule of new drug approval and price listing from January 2025
Considering the current timing of the Drug Committees, the 60/90-day rule from new drug approval to drug price listing, and the explanation given at the Chuikyo on May 15, 2024, the following schedule is expected.
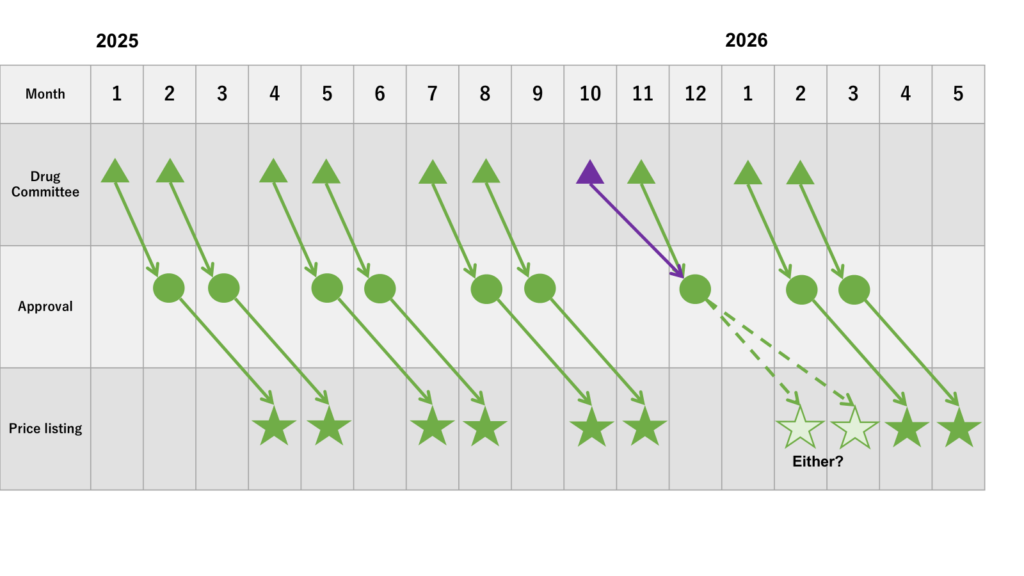
It is assumed that the Drug Committees are held in January, February, April, May, July, August, October, and November, as is the schedule in 2024 or past. Based on this assumption, as the notice indicates that a new drug approval occurs within three weeks of the relevant Drug Committee, the approval is expected to occur the month after the Drug Committee. From that point on, the drug price listing occurs based on the 60-day rule.
However, as stated in the notice, new drugs discussed/reported at all Drug Committees from October to December are to be approved within three weeks of the last committee among them. So, the new drugs discussed in the October committee will be approved 2 months later. It should be noted that this schedule includes the assumptions that no Drug Committees should be held after mid-December.
If a new drug approval is made around December in 2025, there is a high possibility that the drug price listing is to occur not in February 2026 (the 60-day rule), but in March 2026, which would satisfy the 90-day rule. This is because the drug price revision process are highly intensive during January so February 2026.
A specific drug price listing schedule is likely to be presented at the Chuikyo General Assembly once 2025 approaches.


コメント